Welcome to
On Feet Nation
Members
-
bsoftindia Online
-
umar khan Online
-
jack452 Online
-
mark smith Online
-
-
yousuf Online
-
Khalid Shaikh Online
-
Jerry Online
-
Dwayne Online
-
Anna Online
-
Ladhani Metal Corporation Online
-
Jakes lessor Online
Blog Posts
Luxurious Watches: Timeless Beauty and Development in Horology
Posted by Harry on May 15, 2024 at 3:39am 0 Comments 0 Likes
Haute Couture and Sustainable… Continue
Top Content
Do You Need to Register a Device inthe UK? Here's How:
A blog around the process and requirements of registering a medical device in the UK.
If you're planning on marketing a medical device in the UK, and haven't yet familiarized yourself with the registration process for medical devices, it's time to get started.
As a medical device importer or distributor located in the UK, you will have to comply with all relevant UK product regulations, including registration and listing with the Medicines and Healthcare Products Regulatory Agency (MHRA).

The process of registering a medical device will require working with several partners, including the manufacturer and the retailer, assisting in obtaining product safety documentation, completing a self-assessment checklist, and applying for a CTX/Safecode. We'll walk you through each step.Technical File
When it comes to the medical device industry, certain guidelines and restrictions need to be met for the safety and security of patients. From regulating laws, to quality control and safety policies, there are many elements which all have their part to play in ensuring that high standards are kept within the UK.
And one of these elements is registration, a very important element in ensuring your devices is traceable, providing evidence that they have gone through regulatory processes and checks, and allowing you to distribute the product on an international scale.
How to register a medical device in the UK
Medical devices are regulated in the UK by two agencies:
- The Medicines and Healthcare products Regulatory Agency (MHRA).
- The Office for Product Safety and Standards (OPSS).
Before you can sell your medical device in the UK, you must register it with one of these agencies. You'll need to apply for separate registration if your product is both a medical device and a drug.
The process of registering your device varies slightly depending on whether you're an overseas manufacturer or an EU-based manufacturer. However, there are some common steps:
You must have an establishment number, which is issued by OPSS. This number will be part of the registration application form.
You must have a Unique Device Identifier (UDI), which is issued by MHRA. This is usually done at the same time as applying for registration with OPSS.
Registering a medical device with the MHRA
The Medicines and Healthcare products Regulatory Agency (MHRA) is the UK's medicines and medical devices regulator. The MHRA regulates medical devices and drugs, ensuring that they are safe and work as intended.
The MHRA has different requirements for registering a device depending on how you intend to use it. If you're using it in an NHS setting, you won't need to register with the MHRA - but if you're selling or importing it, then it will be required.
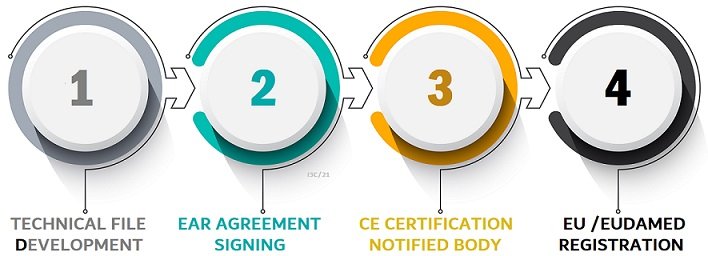
If you're manufacturing or importing a medical device, then you must register with the MHRA. This applies if your company is based in the UK or not - so even if your product will be used in another country, you may still need to register wit
Why I3CGLOBAL
I3CGlobal is the leading medical device registration specialist in the UK. We have over 10 years of experience in this field and have registered over 1,000 different devices for our clients. Our team of experts can help you with any aspect of your medical device registration process, from initial application to final approval.
Read More:- European Authorized Representative
© 2024 Created by PH the vintage.
Powered by
![]()
You need to be a member of On Feet Nation to add comments!
Join On Feet Nation