Welcome to
On Feet Nation
Members
-
jack452 Online
-
jhon a thompson Online
-
Gabriel Online
Blog Posts
AI Watermark Removal: Free Online Solutions and Guidelines
Posted by jack452 on June 15, 2024 at 9:09pm 0 Comments 0 Likes
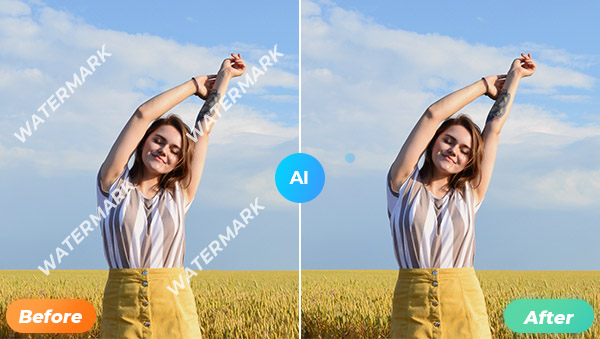
In today's digital age, the proliferation of artificial intelligence (AI) has revolutionized various facets of our lives, including the way we handle digital media. One of the notable advancements is the ability to remove watermarks from images online using AI technologies. This article delves into the intricacies of AI-powered watermark removal, its ethical implications, and how users can access these services for free online.
Understanding…
ContinueDiscover the Excellence of Liss Cream Chargers
Posted by jhon a thompson on June 15, 2024 at 9:08pm 0 Comments 0 Likes
Introduction
In the culinary world, precision and consistency are paramount, especially when it comes to creating perfect whipped cream and other aerated foods. Liss Cream Chargers are renowned for their quality and reliability, making them a preferred… Continue
Top Content
MDD to MDR
IZiel alongwith their partners (Obelis) provide a “One-Stop Shop” to assist medical devicemanufacturers for MDD-MDR transition. IZiel ensures to focus on regulating medical devices throughout its lifecycle rather than focussing on any one stage, which is the need of MDR. The MDR seeks to address these concerns so that the end users do not face major safety or quality issues.
Added by Aniket Chaudhari on February 26, 2020 at 3:36am — No Comments
MDR Consultants
IZiel Healthcare are leading providers of engineering and regulatory services for MDD to MDR projects for medical device companies.IZiel helps you to obtain CE approvals for Class I, II & III Medical Devices, along with MDD to MDR transitions.
Added by Aniket Chaudhari on February 19, 2020 at 4:22am — No Comments
Medical Device Software Validation
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in design and development of highly sophisticated implantable devices at industry leading companies, with direct expertise in software V&V. This team, with the support of IZiel’s regulatory and clinical experts, are decidedly equipped to handle complex software validation activities for…
ContinueAdded by Aniket Chaudhari on February 19, 2020 at 3:54am — No Comments
MDD to MDR
IZiel alongwith their partners (Obelis) provide a “One-Stop Shop” to assist medical devicemanufacturers for MDD-MDR transition. IZiel ensures to focus on regulating medical devices throughout its lifecycle rather than focussing on any one stage, which is the need of MDR. The MDR seeks to address these concerns so that the end users do not face major safety or quality issues.
Added by Aniket Chaudhari on February 13, 2020 at 1:14am — No Comments
MDD to MDR
IZiel alongwith their partners (Obelis) provide a “One-Stop Shop” to assist medical devicemanufacturers for MDD-MDR transition. IZiel ensures to focus on regulating medical devices throughout its lifecycle rather than focussing on any one stage, which is the need of MDR. The MDR seeks to address these concerns so that the end users do not face major safety or quality issues.
Added by Aniket Chaudhari on February 6, 2020 at 7:00am — No Comments
MDD to MDR Consultant
IZiel Healthcare are leading providers of engineering and regulatory services for MDD to MDR projects for medical device companies.IZiel helps you to obtain CE approvals for Class I, II & III Medical Devices, along with MDD to MDR transitions.
Added by Aniket Chaudhari on February 6, 2020 at 6:20am — No Comments
Process Validation for Medical Devices
IZiel Healthcare provides Process Validation for Medical Devices which includes IQ (Installation Qualification), OQ (Operational Qualification), and PQ (Performance Qualification), TMV (Test Method Validation) etc. Our robust Software Validation approach is aligned to IEC 62304 starting with requirements management alongwith risk management & traceability.
Added by Aniket Chaudhari on January 29, 2020 at 11:53pm — No Comments
MDD to MDR
IZiel alongwith their partners (Obelis) provide a “One-Stop Shop” to assist medical devicemanufacturers for MDD-MDR transition. IZiel ensures to focus on regulating medical devices throughout its lifecycle rather than focussing on any one stage, which is the need of MDR. The MDR seeks to address these concerns so that the end users do not face major safety or quality issues.
Added by Aniket Chaudhari on January 24, 2020 at 12:54am — No Comments
MDD to MDR
IZiel alongwith their partners (Obelis) provide a “One-Stop Shop” to assist medical devicemanufacturers for MDD-MDR transition. IZiel ensures to focus on regulating medical devices throughout its lifecycle rather than focussing on any one stage, which is the need of MDR. The MDR seeks to address these concerns so that the end users do not face major safety or quality issues.
Added by Aniket Chaudhari on January 17, 2020 at 2:37am — No Comments
Regulatory Remediation Services
IZiel works in collaboration with your team to develop the complete Design History File (DHF) including requirements management, risk management, process validations and software validations using robust design controls process and quality system procedures. Thereafter, IZiel team works with their regulatory team in USA to complete the submissions (510k or PMA) and resolve queries, if any, from USFDA.…
ContinueAdded by Aniket Chaudhari on January 3, 2020 at 11:30pm — No Comments
Medical Device Software Validation
IZiel has highly trained software engineers with multiple years of experience in software coding, software verification and software validation. The team consists of senior engineers who have worked in design and development of highly sophisticated implantable devices at industry leading companies, with direct expertise in software V&V. This team, with the support of IZiel’s regulatory and clinical experts, are decidedly equipped to handle complex software validation activities for…
ContinueAdded by Aniket Chaudhari on December 27, 2019 at 7:51am — No Comments
FDA Remediation Consultants
Remediation Consultants at IZiel implement best practices in Design-Development Process and Quality Management Systems ensuring effective and timely deliveries. IZiel provides expert solutions to remediate 483 observations and warning letters. IZiel teamworks with medical device companies across the globe to achieve regulatory andstandard compliance.
Added by Aniket Chaudhari on December 20, 2019 at 12:07am — No Comments
Form 483 Compliance
IZiel provides expert solutions to remediate FDA 483 observations and warning letters. IZiel team works with medical device companies across the globe to achieve regulatory and standard compliances. We take great pride in implementing best practices in Design-Development Process and Quality Management Systems ensuring effective and timely deliveries.
Added by Aniket Chaudhari on December 13, 2019 at 6:52am — No Comments
- ‹ Previous
- 1
- …
- 39
- 40
- 41
- Next ›
Latest Blog Posts
Most Popular Blog Posts
Monthly Archives
2024
2023
- December (16)
- November (20)
- October (8)
- September (15)
- August (16)
- July (20)
- June (15)
- May (22)
- April (21)
- March (18)
- February (12)
- January (23)
2022
- December (21)
- November (16)
- October (13)
- September (12)
- August (23)
- July (19)
- June (29)
- May (16)
- April (17)
- March (16)
- February (12)
- January (24)
2021
- December (20)
- November (17)
- October (17)
- September (19)
- August (16)
- July (16)
- June (21)
- May (9)
- April (15)
- March (10)
- February (13)
- January (9)
2020
- December (11)
- November (9)
- October (9)
- September (11)
- August (10)
- July (12)
- June (10)
- May (6)
- April (4)
- March (6)
- February (6)
- January (4)
2019
- December (3)
© 2024 Created by PH the vintage.
Powered by
![]()